Research
"Prediction of novel perovskite-type oxyhydrides"
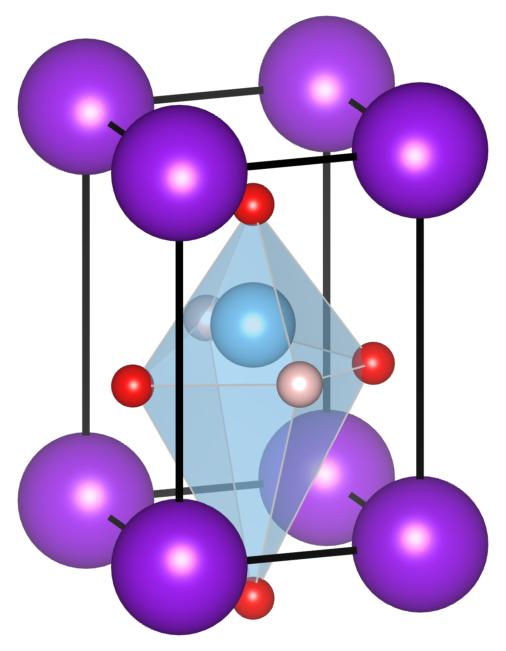
FIG. 1: The crystal structure of KTiO2H. Each color denotes K (purple), Ti (light blue), O (red) and H (pale orange) respectively.
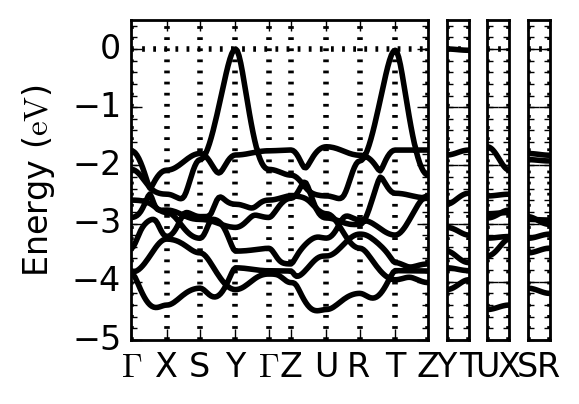
FIG. 2: The valence band of KTiO2H. The dispersion along Y–T is small at the top of the valence band.
Perovskite type oxides ABO3 have been widely used and researched for its piezoelectricity or ferroelectricity. These properties are controlled by substituting A or B cations with other cations like Pb(Zr,Ti)O3 (PZT), while compositions of these cations are limited for the charge neutrality, e.g. A2+B4+O2−3. As well as the substitution of cations, the substitution of oxygen anions (O2−) with halogen anions such as F− has been performed, and recently the substitution with hydrogen anions (H−) has also become available. It suggests that more various compositions of cations might be realized, e.g. A+B4+O2−2H, and they might show the large piezoelectricity, ferroelectricity or peculiar electronic properties.
We investigate an unsynthesized model composition KTiO2H with first-principles calculations as one of such possibilities. This compound is energetically more stable than other structures, showing large electronic polarization comparable with PbTiO3. Moreover, it has two-dimensional electronic states at the top of the valence band characterized as oxygen and hydrogen states [1].
[1] In preparation.