 |
Japanese |
Tsuneyuki Research Group |
Research
"1H NMR chemical shifts of BaTiO3−xHx"
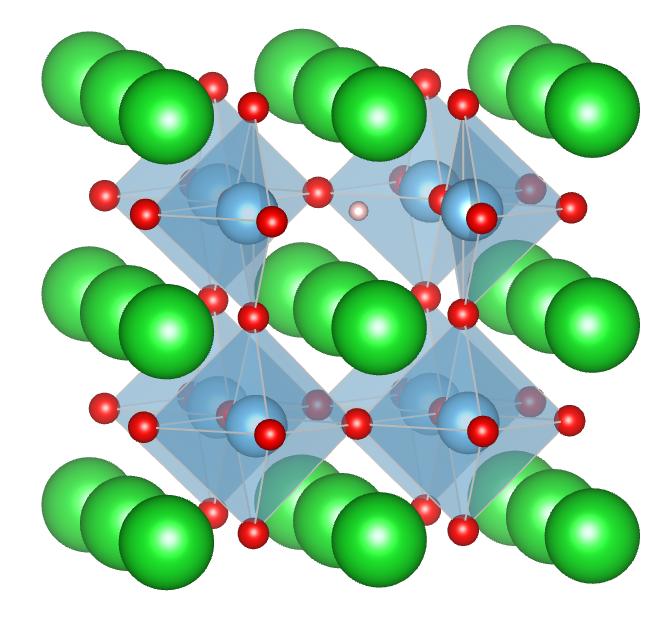
Recently it becomes able to substitute hydride (H−) in a solid for anions in spite of the strong reducing power of hydride. Hydride substitution is utilized for controlling physical properties through such as electron doping. To measure the position or the electronic structure of hydrogen in a solid as above, proton nuclear magnetic resonance (1H NMR) is one of the effective measurement. The 1H NMR chemical shift is affected by the charge state of hydrogen. It is considered to be positive for proton (H+) and negative for hydride (H−), but chemical shifts of hydride in some solids are positive. It is not easy to understand its cause. We target BaTiO3−xHx which is substituted oxygen in barium titanate for hydrogen, and have calculated the charge state and the chemical shift of hydrogen from first-principles and have compared with experiments.
In every hydrogen concentration x = 0.125, 0.5, 1, hydrogen is stable at the oxygen site and the number of electron which hydrogen has is about 1.6 as results of the structure relaxation and the Bader charge analysis. This result is consistent with the experiment. Furthermore we calculate chemical shifts in the insulating state by setting background charges. Resulting values are in the range of 1 to 3 ppm, though they are different with hydrogen concentration. It shows a same tendency to be positive as an experimental value 4.4 ppm on x = 0.1, but the difference between the calculated and the experimental value is larger than that of other materials. This suggests need to consider an effect of conduction electrons.
